Investigating the differences between diesel and gasoline
It is true that diesel and gasoline are both derived from crude oil, but they have different refining methods. Diesel refining is easier than gasoline but produces more pollutants. Diesel produces more energy per liter than gasoline, and therefore makes the combustion of the car engine using it more efficient and emits less CO2. Buying diesel costs less than gasoline and the color of diesel is darker than gasoline. Gasoline is a mixture of alkanes and cycloalkanes obtained by distilling crude oil. The carbon chain length of each gasoline molecule varies from 5 to 12. Gasoline is commonly used in internal combustion engines to increase speed. Gasoline is lighter and produces less pollution than diesel. Diesel is composed of alkanes and carbon chains in general. Each molecule contains a carbon chain 12 carbon atoms long. Diesel is a relatively heavier fuel, with more viscosity and less volatility than gasoline. Diesel is commonly used in combustion engines.
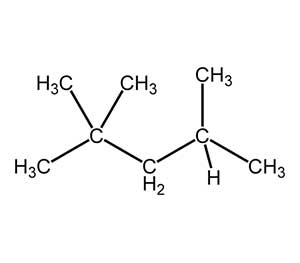
The chemical structure of gasoline
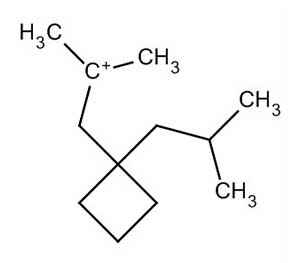
Chemical structure of diesel
chemical mixture: Gasoline is basically made from a combination of hydrocarbons of length 7 (heptane) or 8 (trimethylpentane); Diesel, on the other hand, contains a combination of petroleum hydrocarbons in the range of 8 (octane) to 21 (undecond). The chemical formula of gasoline is C8H18 and the chemical formula of diesel is C12H23.
CO2emissions: Because diesel has a higher percentage of carbon atoms per molecule, it produces approximately 13 percent more gasoline, CO2, or carbon dioxide.
Energycontent: In terms of energy content, diesel is in a higher position than gasoline. The energy content of gasoline is 33.7 megajoules per kilogram and diesel is 36.7 megajoules per kilogram; As a result, diesel fuel is 16% ahead of its competitor in this area.
Viscosity: Gasoline has a lower viscosity than diesel. Of course, as the temperature decreases, the viscosity of diesel increases, but such behavior does not occur in gasoline at limited temperature changes.
Volatility: Due to the lighter nature of gasoline molecules and the presence of some additives, this fuel is more volatile than diesel; This means that at a constant temperature, more gasoline molecules evaporate than diesel. As a result, gasoline ignites faster.
Boiling range : First of all, it should be noted that due to the combined nature of these two fuels, it is not possible to use the term boiling point as a pure substance (eg water); That is, the two boil over the same temperature range . This range is 20 to 150 degrees Celsius for gasoline and 37 to 450 degrees Celsius for diesel.
Fuel consumption: Gasoline is ahead of its rival in this respect; This means that less gasoline is consumed per 100 kilometers than diesel.
Power:Diesel is more powerful than gasoline. The energy production capacity of this fuel is equal to 38.6 megajoules per liter. Another point is that gasoline engines generally produce higher engine speeds. Diesel engines, of course, need more torque.
Fire: Storage more than any fuel needs is undoubtedly dangerous. Gasoline, for example, is known to be a very flammable and dangerous fuel due to its high “vapor pressure” Due to the lower gasoline vapor pressure, this fuel ignites later and is less dangerous during an accident.
Combustion type: The “self-ignition point” of gasoline is in the range of 247 to 280 degrees Celsius. This is the lowest temperature at which a substance ignites spontaneously in a normal atmosphere without any external stimuli such as sparks or flames. Of course, in order to completely burn fuel and oxygen in the car, there is a need for a spark. On the other hand, the self-ignition point of diesel is 210 degrees Celsius and does not need a spark to ignite. Only increasing the fuel pressure is used to cause an explosion. The mixture of fuel and oxygen is compressed together again, which raises the temperature of the mixture to the point of explosion.
Firing point: The “flash point” is the lowest temperature at which the vapor fuel is ready to ignite. At this point there is definitely a need for a flame to ignite. This temperature is calculated at minus 43 degrees Celsius for gasoline and 52-96 degrees Celsius for diesel. At the “fire point” temperature, the fuel vapor burns for at least 5 seconds after starting combustion. This temperature is about 10 degrees higher than the flash point for both fuels.
Classification with octane: Gasoline is measured by “octane number”. The higher this number, the higher the fuel resistance to spontaneous combustion, which causes the engine to soften. Another difference is that diesel is measured by “cetane number”. The larger this number, the faster the fuel ignites in the process of increasing the pressure. The combustion rate of diesel depends on the cetane number.
